Introduction
In the era of miniaturization and personalized healthcare, the integration of magnets into medical devices has become indispensable. From implantable pacemakers and cochlear implants to wearable biosensors and advanced imaging systems, medical-grade magnets are the silent workhorses enabling innovation, precision, and reliability. This comprehensive article explores the science, engineering, safety, and future trends of medical-grade magnets for implantable and wearable devices, with a special focus on design considerations, regulatory compliance, and emerging applications.
—
1. Understanding Medical-Grade Magnets: Materials and Types
1.1 Fundamentals of Magnetism in Medical Devices
Magnets generate a magnetic field due to the movement of electrons within certain materials. In medical technology, the ability to manipulate, sense, and respond to magnetic fields allows for non-invasive diagnostics, targeted therapies, and seamless device integration. The choice of magnet is dictated by requirements such as biocompatibility, field strength, stability, and geometric constraints.
1.2 Common Magnet Types Used in Medical Devices
- Neodymium Iron Boron (NdFeB, or Neodymium) Magnets: Known for their exceptional strength-to-size ratio, neodymium magnets are widely used in applications where compactness and high magnetic force are required. However, they are brittle and prone to corrosion, necessitating robust coatings.
- Samarium Cobalt (SmCo) Magnets: These rare-earth magnets offer slightly lower magnetic strength compared to neodymium but boast superior thermal stability and corrosion resistance, making them ideal for implantable devices.
- Ferrite (Ceramic) Magnets: Less common in implantables due to lower magnetic strength, ferrite magnets are favored in certain sensors and wearable devices for their excellent electrical insulation and cost-effectiveness.
- Bonded Magnets: Created by binding magnetic powders with polymers, these can be intricately molded into complex shapes, enabling custom solutions for miniaturized medical devices and sensors.
1.3 Medical-Grade Requirements for Magnet Materials
Medical-grade magnets must adhere to strict standards for:
- Biocompatibility: They must not elicit an immune response or leach harmful substances.
- Corrosion Resistance: The body’s fluids are highly corrosive, so magnets are encapsulated with biocompatible coatings (e.g., Parylene, gold, titanium).
- Mechanical Integrity: They must withstand mechanical stresses during implantation and operation.
- Magnetic Stability: The magnetic properties must remain stable over the lifespan of the device.
—
2. Design Considerations for Implantable and Wearable Magnets
2.1 Miniaturization and Form Factor
Implantable and wearable devices demand the smallest possible components without sacrifice in performance. The trend toward micro-scale magnets (micromagnets) enables:
- Ultra-thin sensors in biosignal monitoring patches
- Miniature actuators in drug delivery systems
- Custom-shaped magnets for anatomical conformity in implants
Advances in powder metallurgy and injection molding allow for magnets as small as 0.5mm with tight tolerances (±0.01mm), suitable for integration in the most delicate medical applications.
2.2 Magnetic Field Optimization and Directionality
The direction and uniformity of the magnetic field are critical. For example:
- Multi-pole and radial pole magnetization can be used in rotary sensors and actuators for precise feedback or movement.
- Axial magnetization is often chosen for secure coupling in magnetic closures or retention systems (e.g., dental prostheses).
Computer-aided design (CAD) and simulation tools are employed to optimize field strength, minimize stray fields, and ensure safety.
2.3 Coating and Encapsulation
To survive in the challenging environment of the human body, magnets are coated with:
- Parylene: Provides a pinhole-free, biocompatible barrier.
- Gold or Titanium: Used for their inertness and compatibility with tissue.
- Medical-grade polymers: Such as silicone or PTFE, for flexibility and additional protection.
The choice of coating affects both the longevity of the magnet and its potential for integration with other device materials.
2.4 Assembly and Integration
Modern medical device assembly methods include:
- Overmolding: Directly molding the device housing around the magnet for a seamless, hermetic seal.
- Laser welding: For metallic enclosures, ensuring no ingress of fluids.
- Adhesive bonding: Special medical-grade adhesives (such as 3M’s biocompatible lines) are used for reliable fixation.
—
3. Safety and Regulatory Compliance
3.1 Magnetic Interference and Device Compatibility
A major safety concern is the interaction of strong magnets with sensitive medical devices, especially:
- Pacemakers and ICDs (Implantable Cardioverter-Defibrillators): Magnetic fields can inadvertently trigger magnet mode, suspend therapy, or interfere with sensing.
- Other Implants: Cochlear implants, neurostimulators, and insulin pumps may be affected by external magnetic fields.
Strict safety guidelines require:
- Maintaining minimum separation distances between magnets and active implants.
- Screening of patients and staff before MRI procedures.
- Designing magnets with minimal external stray fields through shielding or optimized geometry.
3.2 Handling and Installation Protocols
Due to their strength and brittleness, neodymium and other rare-earth magnets require:
- Use of gloves and eye protection during handling to prevent injury from pinching or flying fragments.
- Secure storage away from metallic tools, electronic devices, and magnetic media.
- Regular inspection for coating integrity, especially for magnets subject to repeated attachment/detachment (e.g., in wearable housings).
3.3 Regulatory Standards
Medical magnets and devices must comply with:
- ISO 10993: Biological evaluation of medical devices (biocompatibility).
- ISO 13485: Quality management for medical device manufacturing.
- IEC 60601-1: Basic safety and performance for medical electrical equipment.
- FDA and CE requirements for risk management, traceability, and post-market surveillance.
Rigorous documentation and testing are mandatory, including cytotoxicity, sensitization, irritation, and long-term stability assessments.
3.4 Special Precautions: Nickel Allergy and Corrosion
Nickel, common in magnetic coatings, can trigger allergic reactions (contact dermatitis) in susceptible individuals. For such cases, hypoallergenic coatings (e.g., gold, Parylene) are mandated, and direct contact with skin is avoided in wearables.
—
4. Core Applications of Medical-Grade Magnets
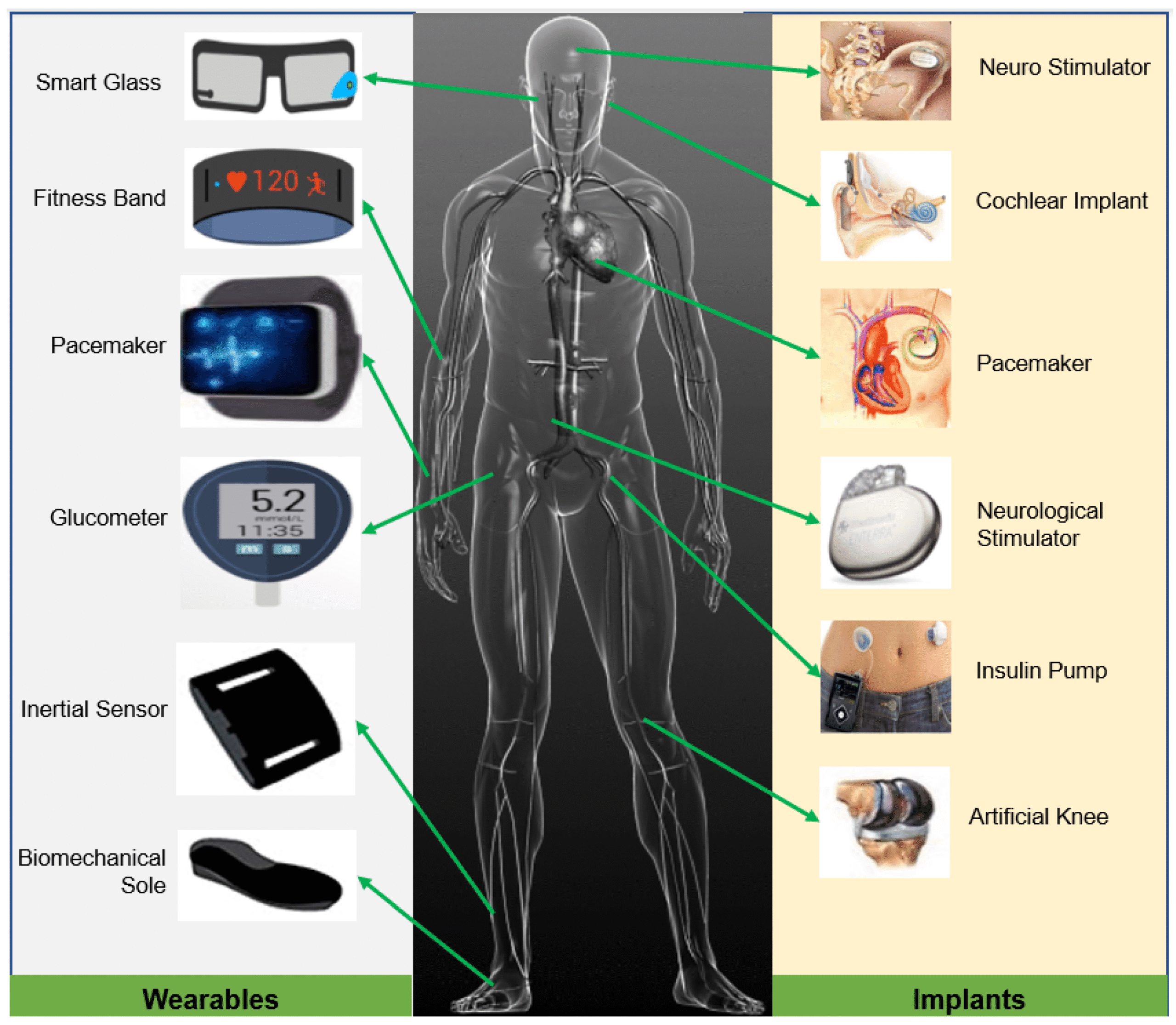
4.1 Implantable Devices
- Pacemakers and ICDs: Magnets are used in reed switches for non-invasive device control and in secure alignment of device components.
- Cochlear Implants: Magnetic coupling enables wireless attachment of external sound processors, facilitating easy removal and cleaning.
- Orthopedic Implants: Magnetic lengthening rods and prostheses utilize controlled magnetic fields for post-surgical adjustments.
- Dental Prostheses: Miniature magnets provide retention in overdentures and maxillofacial prosthetics, improving patient comfort.
4.2 Wearable Medical Devices
- Continuous Glucose Monitors (CGMs): Magnetic snap-fit housings allow for secure, waterproof, and user-friendly sensor attachment.
- Smart Patches and Biosensors: Embedded magnets enable reliable skin contact and alignment, enhancing signal quality and user comfort.
- Magnetic Therapy Devices: Though controversial, some wearable devices use weak static magnetic fields for pain relief and circulation improvement.
4.3 Diagnostic and Therapeutic Systems
- Magnetic Resonance Imaging (MRI): Superconducting electromagnets (typically 1.5T to 3T, up to 7T+ for research) generate powerful, uniform fields essential for high-resolution imaging. These systems rely on precisely engineered magnet assemblies (often using rare-earth elements).
- Targeted Drug Delivery: Magnetic nanoparticles are guided to specific body sites using external magnetic fields, enabling localized therapy with minimal systemic side effects.
- Magnetically Actuated Micro-Robots: Used for minimally invasive diagnostics, targeted biopsies, or drug release in hard-to-reach tissues.
4.4 Microfluidic and MEMS Devices
- Lab-on-a-Chip Systems: Magnetically responsive beads enable the separation, purification, and manipulation of biological samples.
- Microelectromechanical Systems (MEMS): Integration of micro magnets allows for actuation, sensing, and energy harvesting in implantable diagnostics and therapeutic devices.
—
5. Engineering Challenges and Solutions
5.1 Miniaturization Without Compromise
As device footprints shrink, maintaining magnetic performance is increasingly challenging. Solutions include:
- Developing high-energy-density materials (e.g., Grade N56 neodymium magnets) to deliver strong fields in minimal volumes.
- Utilizing advanced manufacturing (e.g., laser micromachining, additive manufacturing) for intricate shapes and assemblies.
5.2 Assembly Integration and Quality Assurance
Magnet assemblies must be robust and reliable over the device lifecycle. Best practices include:
- Overmolding and hermetic sealing to prevent corrosion and physical damage.
- Automated inspection systems (X-ray, CT scan, or magnetic field mapping) to verify placement and orientation.
- Regular performance testing and accelerated aging studies to predict long-term behavior.
5.3 Balancing Magnetic Strength and Safety
Overly strong fields can cause safety hazards or device interference. Engineers use:
- Magnetic shields (e.g., Mu-metal) to contain stray fields.
- Finite element analysis (FEA) to model field distributions and optimize for both efficacy and safety.
—
6. Future Trends in Medical Magnet Technology
6.1 New Materials and Sustainable Manufacturing
Ongoing research aims to develop:
- Corrosion-resistant, high-performance ferrites and rare-earth alternatives for enhanced biocompatibility and reduced environmental impact.
- Eco-friendly manufacturing processes and recyclable coatings to minimize ecological footprint.
6.2 Integration with Smart and Connected Systems
The convergence of magnet technology with the Internet of Things (IoT) and artificial intelligence enables:
- Smart wearables with real-time health monitoring, leveraging magnetic sensors for gesture recognition, positioning, and biosignal detection.
- Wireless power transfer and data communication using magnetically coupled circuits.
6.3 Miniaturized Actuators and Microrobotics
The rise of micromagnets is fueling innovations in:
- Implantable micro-robots for precision surgery and localized therapy.
- Advanced drug delivery systems capable of navigating complex biological environments under external magnetic control.
6.4 Enhanced Customization and Rapid Prototyping
Advanced CAD, simulation, and rapid prototyping tools now allow:
- Tailoring of magnet shapes, field profiles, and coatings to specific patient needs or anatomical locations.
- Shorter development cycles and faster clinical translation for novel devices.
—
7. Best Practices: Installation, Maintenance, and Critical Environment Recommendations
7.1 Installation Guidelines
- Ensure all contact surfaces are clean and free from debris to maintain secure adhesion or mechanical fit.
- Label magnet storage areas and device packaging to warn users and staff of magnetic hazards.
- Integrate magnets only according to validated engineering drawings and process controls.
7.2 Maintenance and Inspection
- Schedule regular testing for magnetic strength, coating integrity, and mechanical wear, especially for devices subjected to frequent use or movement.
- Replace devices immediately if coating breach or corrosion is detected to prevent patient exposure.
7.3 Compliance and Documentation
- Adhere to all relevant industry standards for medical device quality and safety.
- Maintain thorough records of magnet batch numbers, performance tests, and failure investigations.
7.4 Special Recommendations for High-Risk Environments
- Never allow unauthorized personnel to handle strong magnets or magnetized assemblies.
- In MRI suites, rigidly control access and screen all individuals and equipment for ferromagnetic materials.
- Consult with clinical experts and magnetics engineers before introducing new magnet-based technologies to sensitive environments.
—
8. Conclusion: The Expanding Role of Medical-Grade Magnets
Medical-grade magnets are foundational to the next generation of healthcare technology. Their unique properties—high strength in small packages, customizability, and reliability—enable innovations in diagnostics, therapy, and patient monitoring that were once inconceivable. As material science, engineering, and regulatory frameworks continue to evolve, the safety, efficacy, and versatility of these magnets will only increase.
From the high-powered superconducting magnets in MRI scanners to tiny micromagnets in wearable sensors, the future of medicine will be intimately linked to advances in magnetic technology. By adhering to rigorous design, safety, and testing protocols, and by embracing the potential of new materials and smart integration, the medical community can harness the full potential of magnets to improve patient outcomes and shape the healthcare landscape of tomorrow.
—